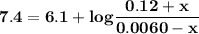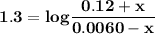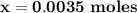Blood is buffered by carbonic acid and the bicarbonate ion. Normal blood plasma is 0.024 Min HCO?3 and 0.0012 M H2CO3 (pKa1 for H2CO3 at body temperature is 6.1).
A. What is the pH of blood plasma?
I got 7.4 for pH which is the correct answer
B. If the volume of blood in a normal adult is 5.0 L, what mass of HCl could be neutralized by the buffering system in blood before the pH fell below 7.0 (which would result in death)?
C. Given the volume from part B, what mass of NaOHcould be neutralized before the pH rose above 7.8?
Express your answer using two significant

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Do you know the correct answer?
Blood is buffered by carbonic acid and the bicarbonate ion. Normal blood plasma is 0.024 Min HCO?3 a...
Questions in other subjects:

Computers and Technology, 18.07.2019 11:50

Business, 18.07.2019 11:50

History, 18.07.2019 11:50


Business, 18.07.2019 11:50

Biology, 18.07.2019 11:50

Biology, 18.07.2019 11:50




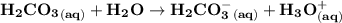
![\mathbf{pH = pKa_1 + log \dfrac{[HCO_3^-]}{[H_2CO_3]}}](/tpl/images/0560/4723/d4625.png)
![\mathbf{pH =6.1 + log \dfrac{[0.024]}{[0.0012]}}](/tpl/images/0560/4723/a7134.png)
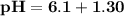
![\mathbf{pH = pKa_1+ log \dfrac{[ salt]}{[acid] }}](/tpl/images/0560/4723/dbb85.png)
![\mathbf{7 =6.1+ log \dfrac{[ 0.024 \times 5 -x]}{[0.0012 \times 5 +x] }}](/tpl/images/0560/4723/43579.png)
![\mathbf{0.9= log \dfrac{[ 0.024 \times 5 -x]}{[0.0012\times 5 +x] }}](/tpl/images/0560/4723/2dcb7.png)
![\mathbf{10^{0.9}= \dfrac{[ 0.024 \times 5 -x]}{[0.0012 \times 5 +x] }}](/tpl/images/0560/4723/8672b.png)
![\mathbf{7.94 = \dfrac{[ 0.024 \times 5 -x]}{[0.0012 \times 5 +x] }}](/tpl/images/0560/4723/b837c.png)
![\mathbf{7.94 = \dfrac{[ 0.12-x]}{[0.006 +x] }}](/tpl/images/0560/4723/81a5e.png)
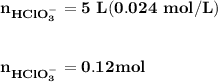
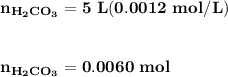
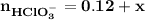
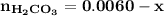
![\mathbf{pH = pKa_1+ log \dfrac{[ HCO_3^-]}{[H_2CO_3] }}](/tpl/images/0560/4723/f8866.png)