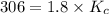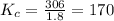Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, sdlesley66
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 21:20, 50057543
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2


Chemistry, 23.06.2019 07:20, msladycie8831
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1
Do you know the correct answer?
Carbon monoxide replaces oxygen in oxygenated hemoglobin according to the reaction: HbO2(aq) + CO(aq...
Questions in other subjects:

Mathematics, 26.11.2020 03:50



History, 26.11.2020 04:00

Physics, 26.11.2020 04:00


Mathematics, 26.11.2020 04:00

Mathematics, 26.11.2020 04:00


Social Studies, 26.11.2020 04:00


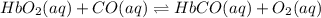
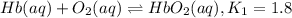
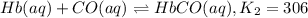
![K_1=\frac{[HbO_2]}{[Hb][O_2]}](/tpl/images/0560/2528/dcc99.png)
![[Hb]=\frac{[HbO_2]}{[K_1][O_2]}](/tpl/images/0560/2528/1a6fb.png) ..[1]
..[1]![K_2=\frac{[HbCO]}{[Hb][CO]}](/tpl/images/0560/2528/abc0a.png) ..[2]
..[2]![K_c=\frac{[HbCO][O_2]}{[HbO_2][CO]}](/tpl/images/0560/2528/bc291.png) ..[3]
..[3]![K_2=\frac{[HbCO]}{\frac{[HbO_2]}{[K_1][O_2]}\times [CO]}](/tpl/images/0560/2528/e05b3.png)
![K_2=K_1\times \frac{[HbCO][O_2]}{[HbO_2][CO]}](/tpl/images/0560/2528/e36c4.png)
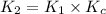 ( using [3])
( using [3])