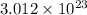Chemistry, 24.03.2020 01:27, juanesmania
When an ionic compound such as sodium chloride (NaCl) is placed in water the component atoms of the NaCl crystal dissociate into individual sodium ions (Na+) and chloride ions (Cl-). In contrast, the atoms of covalently bonded molecules (e. g., glucose, sucrose, glycerol) do not generally dissociate when placed in aqueous solution. Which of the following solutions would be expected to contain the greatest number of particles (molecules or ions)? A) 1 L of 0.5 M NaCl B) 1 L of 0.5 M glucose C) 1 L of 1.0 M NaCl D) 1 L of 1.0 M glucose E) C and D will contain equal numbers of particles.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1


Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
Do you know the correct answer?
When an ionic compound such as sodium chloride (NaCl) is placed in water the component atoms of the...
Questions in other subjects:


Chemistry, 23.01.2020 08:31

History, 23.01.2020 08:31



Mathematics, 23.01.2020 08:31




History, 23.01.2020 08:31


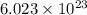 number of molecules/ions
number of molecules/ions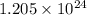 number of ions
number of ions volume) no. of particles
volume) no. of particles