
Chemistry, 24.03.2020 00:56, arielmajesty9107
The two reactions involved in quantitatively determining the amount of iodate in solution are: IO3-(aq) + 5 I-(aq) + 6 H+(aq) --> 3 I2(aq) + 3 H2O(l) followed by reaction of the I2: I2(aq) + 2 S2O32- --> 2 I-(aq) + S4O62-(aq). What is the stoichiometric factor, that is the number of moles of Na2S2O3 reacting with one mole of KIO3?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, latoyatuggle23
Endeleev saw trends in the physical and chemical properties of elements when he organized them by
Answers: 2


Chemistry, 22.06.2019 14:30, hjlhdjfhjh
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 19:00, elizabethajih99
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
Do you know the correct answer?
The two reactions involved in quantitatively determining the amount of iodate in solution are: IO3-(...
Questions in other subjects:


Social Studies, 22.08.2019 04:50

History, 22.08.2019 04:50


Mathematics, 22.08.2019 04:50






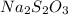 reacting with one mole of
reacting with one mole of 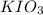 .
.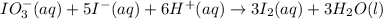 ..[1]
..[1]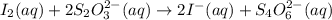 ..[2]
..[2]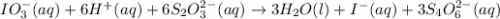
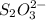 reacts with 1 mole of
reacts with 1 mole of 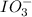 .
.



