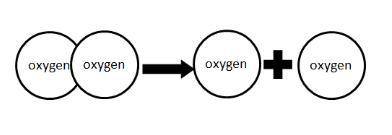
As we move from the left side of the arrow to the right, the two oxygen atoms exhibit
A) more...

Chemistry, 24.03.2020 00:31, lewisf2578
As we move from the left side of the arrow to the right, the two oxygen atoms exhibit
A) more stability.
B) higher entropy.
C) less potential energy.
D) a decrease in temperature.
IMAGE SHOWN BELOW.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, suzymott1562
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1

Chemistry, 21.06.2019 16:00, sammiehammer
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 21.06.2019 17:30, sophiebeardsley94
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Mathematics, 02.02.2021 06:30


Mathematics, 02.02.2021 06:30

Mathematics, 02.02.2021 06:30

Mathematics, 02.02.2021 06:30




Social Studies, 02.02.2021 06:30






