
A 18.08-g sample of the ionic compound , where is the anion of a weak acid, was dissolved in enough water to make 116.0 mL of solution and was then titrated with 0.140 M . After 500.0 mL was added, the pH was 4.63. The experimenter found that 1.00 L of 0.140 M was required to reach the stoichiometric point of the titration. a What is the molar mass of ? Molar mass = 129.14 g/mol b Calculate the pH of the solution at the stoichiometric point of the titration.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, jmanrules200
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 21.06.2019 21:30, gatorr2010
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 21.06.2019 22:30, mauifrifer3986
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 02:40, hardwick744
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2
Do you know the correct answer?
A 18.08-g sample of the ionic compound , where is the anion of a weak acid, was dissolved in enough...
Questions in other subjects:

Mathematics, 18.10.2019 07:50

Mathematics, 18.10.2019 07:50


Mathematics, 18.10.2019 07:50

English, 18.10.2019 07:50



Mathematics, 18.10.2019 07:50


Spanish, 18.10.2019 07:50


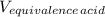 = 1.00 L
= 1.00 L
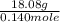
![10^{-4.63]](/tpl/images/0559/8018/9ece0.png)
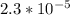
![[A^-]equ = \frac{0.140M*1.00L}{1.00L+0.116L}](/tpl/images/0559/8018/a9cf4.png)
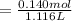
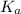 of HA =
of HA = 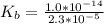
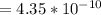
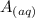 +
+ 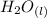
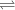
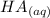 +
+ 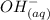
![K_b = \frac{[HA][OH^-]}{[A^-]}](/tpl/images/0559/8018/12da8.png)
![4.35*10^{-10} = \frac{[x][x]}{[0.1255-x]}](/tpl/images/0559/8018/ab7b7.png)
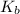 is very small, (o.1255 - x) = 0.1255
is very small, (o.1255 - x) = 0.1255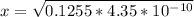
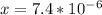
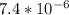 ]
]



