
Chemistry, 23.03.2020 21:45, angelina12386
For cobalt, Co, the heat of vaporization at its normal boiling point of 3097 °C is 389.1 kJ/mol. The entropy change when 1.85 moles of liquid Co vaporizes at 3097 °C, 1 atm is J/K.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3
Do you know the correct answer?
For cobalt, Co, the heat of vaporization at its normal boiling point of 3097 °C is 389.1 kJ/mol. The...
Questions in other subjects:

Mathematics, 20.09.2019 13:50




Chemistry, 20.09.2019 14:00

Biology, 20.09.2019 14:00


History, 20.09.2019 14:00


History, 20.09.2019 14:00


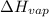 =
= 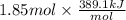
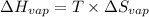
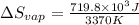
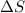 for vaporization is 213.6 J/K.
for vaporization is 213.6 J/K.



