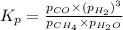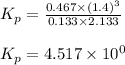Chemistry, 23.03.2020 21:09, darkskinnednune
Steam reforming of methane ( CH4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 1.5 L flask with 0.60 atm of methane gas and 2.6 atm of water vapor at 47. °C. He then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of hydrogen gas to be 1.4 atm. Calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits x10.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, claudr03
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 23.06.2019 00:30, zaniathomasel
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
Do you know the correct answer?
Steam reforming of methane ( CH4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hy...
Questions in other subjects:




Mathematics, 30.03.2021 18:50




Mathematics, 30.03.2021 18:50




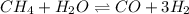
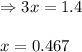
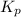 for above equation follows:
for above equation follows: