
Chemistry, 23.03.2020 21:11, copelandgarret9972
What concentration of Cl2O remains after a mixture that initially contains [H2O] = 1.00 M and [Cl2O] = 1.00 M comes to equilibrium at 25 °C ? Kc for the reaction is 0.0900.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2


Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 23.06.2019 02:00, bagofmud8339
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
Do you know the correct answer?
What concentration of Cl2O remains after a mixture that initially contains [H2O] = 1.00 M and [Cl2O]...
Questions in other subjects:

History, 16.10.2021 01:00

Mathematics, 16.10.2021 01:00

Mathematics, 16.10.2021 01:00

Mathematics, 16.10.2021 01:00

Social Studies, 16.10.2021 01:00

Mathematics, 16.10.2021 01:00



Mathematics, 16.10.2021 01:00


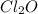 is 0.870 M.
is 0.870 M.![[H_2O]=1.00 M](/tpl/images/0559/4667/b0041.png)
![Cl_2O=[Cl_2O]=1.00 M](/tpl/images/0559/4667/4c14a.png)
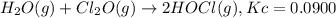
![K_c=\frac{[HOCl]^2}{[H_2O][Cl_2O]}](/tpl/images/0559/4667/da783.png)
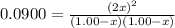
![[Cl_2O]=(1,00-x) M=1.00 M-0.130 M=0.870 M](/tpl/images/0559/4667/c9bd4.png)




