
Chemistry, 23.03.2020 20:21, ranaawilliamsoowl6dk
Consider the reaction represented by the equation?2SO2(g) + O2(g) 2SO3(g).?For the system at chemical equilibrium, which of the following explains what happens after the volume of the reaction mixture is increased (assume constant temperature)?
a. The amount of SO3(g) increases and the value for K increases.
b. The amount of SO3(g) decreases and the value for K increases.
c. The amount of SO3(g) stays the same and the value for K decreases.
d. The amount of SO3(g) decreases and the value for K stays the same.
e. The amount of SO3(g) increases and the value for K stays the same

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 21:00, itasykamila
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
Do you know the correct answer?
Consider the reaction represented by the equation?2SO2(g) + O2(g) 2SO3(g).?For the system at chemica...
Questions in other subjects:











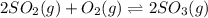
![K=\frac{[SO_3]_{eq}^2}{[SO_2]_{eq}^2[O_2]_{eq}}](/tpl/images/0559/3324/74387.png)




