
Chemistry, 23.03.2020 20:26, kcutler8603
Stearic acid, fatty acid commonly found in animal fat has a condensed Chemical formula CH3(CH2)16COOH. It is a common component of soaps and detergents. Which of the following statements is false?
A The -COOH group would experience hydrogen bonding with water
B The long alkane ''tail" CH3(CH2)16 -, is hydrophbic
C Stearic acid and water are probably miscible
D Stearic acid would probably dissolve in non polar solvents such as hexane C6H14
E Stearic acid contains hydrophobic regions

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, vivianni0727p1y30v
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 21:30, rileydavidharless
Which substance can be broken down by chemical means
Answers: 1

Chemistry, 22.06.2019 23:30, shukriabdisabrie
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 22.06.2019 23:30, johnnysteeler9934
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
Do you know the correct answer?
Stearic acid, fatty acid commonly found in animal fat has a condensed Chemical formula CH3(CH2)16COO...
Questions in other subjects:





Social Studies, 14.07.2019 18:30



Mathematics, 14.07.2019 18:30


History, 14.07.2019 18:30


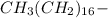 , is hydrophobic.
, is hydrophobic.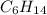 .
.



