Chemistry, 23.03.2020 20:01, lpssprinklezlps
Suppose of iron(II) bromide is dissolved in of a aqueous solution of silver nitrate. Calculate the final molarity of bromide anion in the solution. You can assume the volume of the solution doesn't change when the iron(II) bromide is dissolved in it. Round your answer to significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, lydiadmanautou04
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 23.06.2019 00:30, DragonLovely
•hydration •dissociation •dissolving which one goes to which
Answers: 1
Do you know the correct answer?
Suppose of iron(II) bromide is dissolved in of a aqueous solution of silver nitrate. Calculate the f...
Questions in other subjects:

Business, 26.08.2019 21:10



Law, 26.08.2019 21:10






History, 26.08.2019 21:10


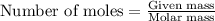
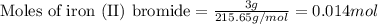
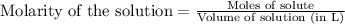 .....(1)
.....(1)
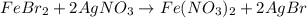
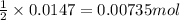 of iron (II) bromide
of iron (II) bromide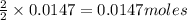 of silver bromide
of silver bromide