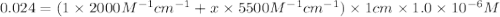The molar absorptivity of a tyrosine residue at 280 nm is 2000 M-1cm-1, while for tryptophan it is 5500 M-1cm-1. A protein has been isolated that is known to contain one tyrosine residue and an unknown number of tryptophans. A 1.0 micromolar solution of this protein is placed in a 1.0 cm cuvette and the absorbance at 280 nm is measured as 0.024. How many tryptophans are in the protein

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 09:20, annapittbull12
1) a. water molecule breaks up into hydrogen and oxygen on passing electricity. does this involve breaking intermolecular or intramolecular forces of attraction. explain b. on boiling water changes to water vapor. does this involve breaking intermolecular or intramolecular forces of attraction. explain methanol evaporates faster than water. contrast the intermolecular forces and the vapor pressures of methanol and water?
Answers: 2
Do you know the correct answer?
The molar absorptivity of a tyrosine residue at 280 nm is 2000 M-1cm-1, while for tryptophan it is 5...
Questions in other subjects:



History, 28.10.2019 20:31



English, 28.10.2019 20:31

Physics, 28.10.2019 20:31

Mathematics, 28.10.2019 20:31


History, 28.10.2019 20:31





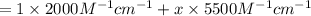
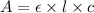 ( Beer-Lambert's law)
( Beer-Lambert's law)