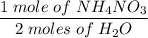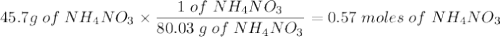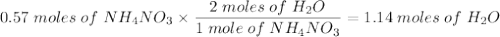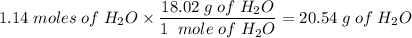Chemistry, 23.03.2020 19:30, shyshy1791
Ammonium nitrate decomposes to dinitrogen monoxide and water. If given 45.7 grams of ammonium
nitrate, how many grams of water would be produced?
NH4NO3(s)—N2O(g)+2H20(1)
0

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
Do you know the correct answer?
Ammonium nitrate decomposes to dinitrogen monoxide and water. If given 45.7 grams of ammonium
...
...
Questions in other subjects:


Mathematics, 21.11.2020 01:20



Biology, 21.11.2020 01:20

English, 21.11.2020 01:20

History, 21.11.2020 01:20