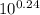that's just how they are
explanation:
you can't control another persons decisions, all you can do is ask that they respect yours. relationships are hard because you have to put the effort into making sure that another person feels seen, heard, and loved. as time goes on, it gets easier, you learn how to do these things without thinking. loving them becomes as easy as breathing, but every relationship will have fights, and sometimes it'll feel like you're drowning, but as long as you can talk it out and compromise, because that's truly what loving another person is, is compromise, then you'll be okay. movies and tv shows have glamorized and romanticized (pardon the pun) what a relationship would be like. real relationships aren't perfectly timed kisses in the rain, zero fights until one big one ends everything, 6 month anniversaries. real relationships are sleepy confused kisses when one person has to leave for work earlier than the other, real relationships are having small disagreements, real relationships are messy and confusing, real relationships is knowing that even if you're relationship may not last forever and this may not be the person you're going to spend the rest of your life with, that you love them, and you would do anything to see them happy. so yes, relationships are difficult, but so are many other things, relationships can be amazing but also stressful, and not just romantic i'm also talking about platonic relationships (although the primary example here was romantic) sometimes is best to leave friendships behind, so remember, always do whats going to you be happy in the long run.

















![\frac{[Base]}{[Acid]}](/tpl/images/0559/1224/ebefd.png) = 1.737
= 1.737