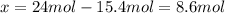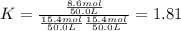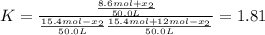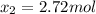While ethanol (CH3CH2OH is produced naturally by fermentation, e. g. in beer- and wine-making, industrially it is synthesized by reacting ethylene CH2CH2) with water vapor at elevated temperatures. A chemical engineer studying this reaction fills a 50.0 L tank at 22. °C with 24. mol of ethylene gas and 24. mol of water vapor. He then raises the temperature considerably, and when the mixture has come to equilibrium determines that it contains 15.4 mol of ethylene gas and 15.4 mol of water vapor The engineer then adds another 12. mol of water, and allows the mixture to come to equilibrium again. Calculate the moles of ethanol after equilibrium is reached the second time. Round your answer to 2 significant digits.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2
Do you know the correct answer?
While ethanol (CH3CH2OH is produced naturally by fermentation, e. g. in beer- and wine-making, indus...
Questions in other subjects:

Physics, 24.10.2021 21:30



Mathematics, 24.10.2021 21:30

History, 24.10.2021 21:30


Mathematics, 24.10.2021 21:30



History, 24.10.2021 21:30


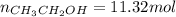
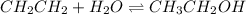
![K=\frac{[CH_3CH_2OH]_{eq}}{[CH_2CH_2]_{eq}[H_2O]_{eq}}](/tpl/images/0559/0707/4b24a.png)
 result:
result: