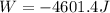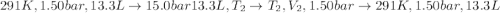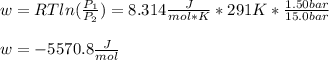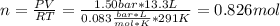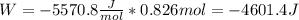An ideal gas described by Ti=291K, Pi=1.50bar, and Vi=13.3L is heated at constant volume until P=15.0bar. It then undergoes a reversible isothermal expansion until P=1.50bar. It is then restored to its original state by the extraction of heat at constant pressure. Calculate w for step 2 (P, Vi, T → Pi, V2, T).

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, Queenquestion5967
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3


Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1
Do you know the correct answer?
An ideal gas described by Ti=291K, Pi=1.50bar, and Vi=13.3L is heated at constant volume until P=15....
Questions in other subjects:

History, 08.11.2020 06:10

Chemistry, 08.11.2020 06:10

Mathematics, 08.11.2020 06:10

History, 08.11.2020 06:10

Mathematics, 08.11.2020 06:10



English, 08.11.2020 06:10

Biology, 08.11.2020 06:10