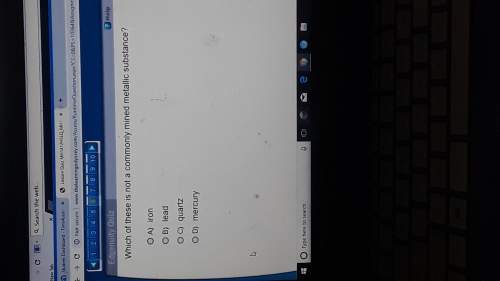
Chemistry, 22.03.2020 19:18, jazmaine1217
Sodium nitrite, NaNO2, is used as a preservative in meat products such as frankfurters and bologna. In an acidic solution, nitrite ion is converted to nitrous acid, HNO2, which reacts with permanganate ion according to the equation:
H++ 5HNO2+ 2MnO4– right-arrow 5NO3 + 2Mn2++ 3H2O
A 1.000 g sample of a water-soluble solid containing NaNO2 was dissolved in dilute H2SO4 and titrated with 0.01000 M KMnO4 solution. The titration required 12.15 mL of the KMnO4 solution. There are 0.0001215 moles of KMnO4.
Using the balanced equation, find the number of moles of HNO2 in the sample.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1


Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 08:00, danielhall
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1
Do you know the correct answer?
Sodium nitrite, NaNO2, is used as a preservative in meat products such as frankfurters and bologna....
Questions in other subjects:


Health, 20.10.2020 05:01

Mathematics, 20.10.2020 05:01


Mathematics, 20.10.2020 05:01

History, 20.10.2020 05:01


English, 20.10.2020 05:01


Mathematics, 20.10.2020 05:01