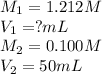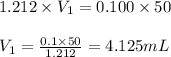Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, pressure772
Which is a character of nuclear fusion but not nuclear fission
Answers: 3


Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1
Do you know the correct answer?
You have a 1.212 M stock aqueous solution of Sr(OH)2. What volume of thestock solution (in mL) must...
Questions in other subjects:



Mathematics, 04.02.2021 22:50

Mathematics, 04.02.2021 22:50

Biology, 04.02.2021 22:50






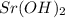 produces 1 mole of strontium ions and 2 moles of hydroxide ions
produces 1 mole of strontium ions and 2 moles of hydroxide ions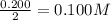
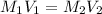
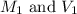 are the molarity and volume of the concentrated strontium hydroxide solution
are the molarity and volume of the concentrated strontium hydroxide solution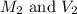 are the molarity and volume of diluted strontium hydroxide solution
are the molarity and volume of diluted strontium hydroxide solution