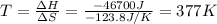Chemistry, 21.03.2020 05:35, milkshakegrande101
For a particular reaction, Δ H ∘ = − 46.7 kJ and Δ S ∘ = − 123.8 J/K. Assuming these values change very little with temperature, at what temperature does the reaction change from nonspontaneous to spontaneous?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 16:30, danbelucio
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
Do you know the correct answer?
For a particular reaction, Δ H ∘ = − 46.7 kJ and Δ S ∘ = − 123.8 J/K. Assuming these values change v...
Questions in other subjects:

Mathematics, 16.07.2019 12:20


Mathematics, 16.07.2019 12:20

Physics, 16.07.2019 12:20

Mathematics, 16.07.2019 12:20



Mathematics, 16.07.2019 12:20



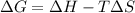
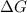 = Gibbs free energy
= Gibbs free energy 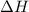 = enthalpy change = -46.7 kJ= -46700 J
= enthalpy change = -46.7 kJ= -46700 J = entropy change = -123.8 J/K
= entropy change = -123.8 J/K