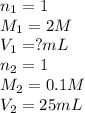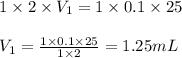Chemistry, 21.03.2020 05:05, Kittylover65
What volume (mL) of the partially neutralized stomach acid was neutralized by NaOH during the titration? (portion of 25.00 mL sample; this was the HCl remaining after the antacid tablet did it's job)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 23.06.2019 01:30, sanchezvazquez0123
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1


Chemistry, 23.06.2019 10:30, malum2009
Can anyone explain 1. review your spectrometry data and use the known elements to identify the star's composition. which unknown elements make up this star? justify your element selections. 2. in parts i and ii of the lab, what happened to the electrons of each element to produce the different colors of light? explain your answers using important terms from the lesson and information provided in the laboratory. 3. stars composed of heavier (more massive) elements are often slightly older than stars made predominantly from hydrogen and helium. based on your data, is the newly discovered star a younger star? explain your answer.
Answers: 2
Do you know the correct answer?
What volume (mL) of the partially neutralized stomach acid was neutralized by NaOH during the titrat...
Questions in other subjects:

History, 05.11.2020 01:20



English, 05.11.2020 01:20




Mathematics, 05.11.2020 01:20

Chemistry, 05.11.2020 01:20

Mathematics, 05.11.2020 01:20


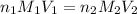
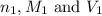 are the n-factor, molarity and volume of stomach acid which is HCl
are the n-factor, molarity and volume of stomach acid which is HCl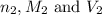 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.