
Chemistry, 21.03.2020 04:33, samantha9014
The colorless gas phosphorus trifluoride reacts slowly with water to give a mixture of phosphorus acid and hydrofluoric acid . (a) Write a balanced chemical equation for this reaction. + + (b) Determine the concentration (in moles per liter) of each of the acids that result from the complete reaction of 5.71×10-2 moles of phosphorus trifluoride with enough water to give a solution volume of 711 mL. phosphorus acid : M hydrofluoric acid : M

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 14:30, joejoefofana
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀ pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4. 0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Do you know the correct answer?
The colorless gas phosphorus trifluoride reacts slowly with water to give a mixture of phosphorus ac...
Questions in other subjects:

Mathematics, 27.09.2019 11:00



Business, 27.09.2019 11:00

English, 27.09.2019 11:00

History, 27.09.2019 11:00

History, 27.09.2019 11:00

Biology, 27.09.2019 11:00


History, 27.09.2019 11:00


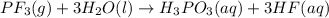
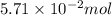
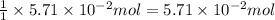 of phosphorus acid
of phosphorus acid![[concentration]=\frac{Moles}{Volume(L)}](/tpl/images/0557/3028/5aba9.png)
![[H_3PO_3]=\frac{5.71\times 10^{-2} mol}{0.711 L}=0.0803 M](/tpl/images/0557/3028/5fed3.png)
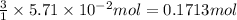 of hydrofluoric acid
of hydrofluoric acid![[HF]=\frac{0.1713 mol}{0.711 L}=0.241 M](/tpl/images/0557/3028/4f9ef.png)




