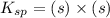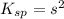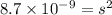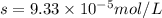Chemistry, 21.03.2020 03:31, samanthasheets8925
Calculate the solubility of ( = ) in moles per liter. Ignore any acid–base properties. s = mol/L Calculate the solubility of ( = ) in moles per liter. Ignore any acid–base properties. s = mol/L Calculate the solubility of ( = ) in moles per liter. Ignore any acid–base properties. s = mol/L

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 23.06.2019 04:00, izzyp619
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
Do you know the correct answer?
Calculate the solubility of ( = ) in moles per liter. Ignore any acid–base properties. s = mol/L Cal...
Questions in other subjects:

Mathematics, 05.05.2020 22:58


History, 05.05.2020 22:58


Biology, 05.05.2020 22:58


Spanish, 05.05.2020 22:58




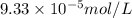
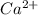 ion and
ion and 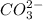 ion.
ion.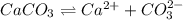
![K_{sp}=[Ca^{2+}][CO_3^{2-}]](/tpl/images/0557/2415/6c60d.png)