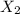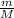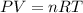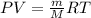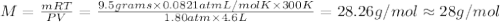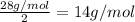To identify a diatomic gas (X2), a researcher carried out the following experiment: She weighed an empty 4.6-L bulb, then filled it with the gas at 1.80 atm and 27.0 ∘C and weighed it again. The difference in mass was 9.5 g . Identify the gas. Express your answer as a chemical formula. View Available Hint(s)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 20:30, ShahinF7536
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
Do you know the correct answer?
To identify a diatomic gas (X2), a researcher carried out the following experiment: She weighed an e...
Questions in other subjects:

Mathematics, 10.03.2021 16:30

Mathematics, 10.03.2021 16:30



Mathematics, 10.03.2021 16:30

Mathematics, 10.03.2021 16:30

English, 10.03.2021 16:30




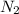 .
.