
Pure copper may be produced by the reaction of copper(I) sulfide with oxygen gas as follows: Cu2S(s) + O2(g) 2Cu(s) + SO2(g) If 0.680 kg of copper(I) sulfide reacts with excess oxygen, what mass of copper metal may be produced ? A) 0.680 kg B) 0.136 kg C) 0.271 kg D) 0.543 kg E) 1.36 kg

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 19:50, strawberrymrmr756
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 22:30, jaylenmiller437
The diagram shows the relationship between scientific disciplines. the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a. physics b. biology c. chemistry d. metallurgy
Answers: 2
Do you know the correct answer?
Pure copper may be produced by the reaction of copper(I) sulfide with oxygen gas as follows: Cu2S(s)...
Questions in other subjects:



English, 25.02.2021 22:20



Health, 25.02.2021 22:20


English, 25.02.2021 22:20



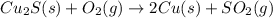
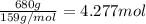
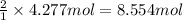 copper
copper



