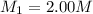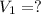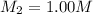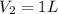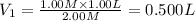A student wants to prepare 1.00 L of a 1.00 M solution of NaOH (molar mass 40.00 g/mol). If solid NaOH is available, how would the student prepare this solution? If 2.00 MNaOH is avail- able, how would the student prepare the solution? To help insure three significant figures in the NaOH molarity, to how many sig- nificant figures should the volumes and mass be determined?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 19:30, mazielynn84
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Do you know the correct answer?
A student wants to prepare 1.00 L of a 1.00 M solution of NaOH (molar mass 40.00 g/mol). If solid Na...
Questions in other subjects:


Biology, 29.01.2020 02:44

Mathematics, 29.01.2020 02:44

Chemistry, 29.01.2020 02:44

Mathematics, 29.01.2020 02:44

Social Studies, 29.01.2020 02:44




Mathematics, 29.01.2020 02:44


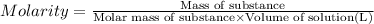
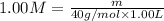
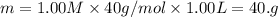
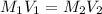 (dilution equation)
(dilution equation)