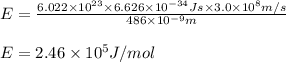Chemistry, 20.03.2020 11:30, vanessa23272
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm? (c = 3.00 × 108 m/s; h = 6.63 × 10–34 J • s; NA = 6.022 × 1023 moles–1)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, luhmimi17
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 19:30, liyahlanderson2232
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
Do you know the correct answer?
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm...
Questions in other subjects:

Biology, 22.06.2019 16:30







History, 22.06.2019 16:30

History, 22.06.2019 16:30


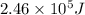
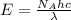
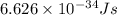
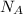 = Avogadro's number =
= Avogadro's number = 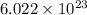
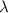 = wavelength of photon = 486 nm =
= wavelength of photon = 486 nm = 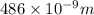 (Conversion factor:
(Conversion factor: 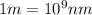 )
)