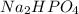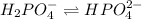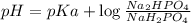Chemistry, 20.03.2020 11:19, MarishaTucker
Buffer consists of 0.50 M NaH2PO4 and 0.40 M Na2HPO4. Phosphoric acid is a triprotic acid (K a 1 = 7.2 × 10 − 3, K a 2 = 6.3 × 10 − 8, and K a 3 = 4.2 × 10 − 13). (a) Which Ka value is most important to this buffer? (b) What is the buffer pH?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, jaylanmahone223
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
Do you know the correct answer?
Buffer consists of 0.50 M NaH2PO4 and 0.40 M Na2HPO4. Phosphoric acid is a triprotic acid (K a 1 = 7...
Questions in other subjects:


Mathematics, 03.06.2021 18:50

History, 03.06.2021 18:50

Mathematics, 03.06.2021 18:50

English, 03.06.2021 18:50


Mathematics, 03.06.2021 18:50


Mathematics, 03.06.2021 18:50


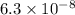 pKa2 = 7.2
pKa2 = 7.2 and
and