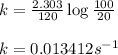Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 10:00, zionlopez543
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1
Do you know the correct answer?
For a first-order reaction, after 2.00 min, 20% of the reactants remain. Calculate the rate constant...
Questions in other subjects:

Biology, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00

Biology, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00



Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00


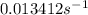
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0556/0145/f1041.png)
![[A_o]](/tpl/images/0556/0145/dc622.png) = initial amount of the sample = 100 grams
= initial amount of the sample = 100 grams