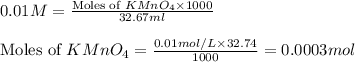Chemistry, 20.03.2020 10:46, jdisalle476
A sample of an Iron Oxalato complex salt weighting 0.13 grams requires 32.74 mL of 0.01 M KMnO4 to turn the solution a very light pink color at the quivalence point. Calculate the number of moles of KMnO4 added.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, Maryjasmine8001
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2

Chemistry, 22.06.2019 13:00, rome58
Lab reagent, hypothesis test. a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl. these six measurements are assumed to be an srs of all possible measurements from solution. they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution. carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 20:00, montimcdaniel
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 23.06.2019 01:00, Zachgrainger4436
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
Do you know the correct answer?
A sample of an Iron Oxalato complex salt weighting 0.13 grams requires 32.74 mL of 0.01 M KMnO4 to t...
Questions in other subjects:

Chemistry, 18.11.2020 22:20

Biology, 18.11.2020 22:20

Advanced Placement (AP), 18.11.2020 22:20

Mathematics, 18.11.2020 22:20

Mathematics, 18.11.2020 22:20

History, 18.11.2020 22:20


Mathematics, 18.11.2020 22:20

Mathematics, 18.11.2020 22:20

English, 18.11.2020 22:20


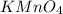 added are 0.0003
added are 0.0003 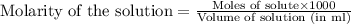 .....(1)
.....(1)