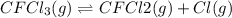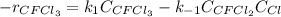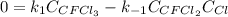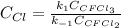Chemistry, 20.03.2020 10:54, diwashkandel6pe02af
Consider the following elementary reaction: CFC13(g)-CFC12(9)+Clg) Suppose we let k1 stand for the rate constant of this reaction, and k1 stand for the rate constant of the reverse reaction Write an expression that gives the equilibrium concentration of Cl in terms of k, k_1, and the equilibrium concentrations of CFCI3 and CFCI2 1. K-1 [ci]

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, kamkam5791
Is powdered sports drink ionic or covalent ? 10pts !
Answers: 1

Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1


Chemistry, 23.06.2019 15:00, alondrabdiaz586
This is a portion of the earths surface that may be far from tectonic plates boundaries yet experiences volcanism due to a rising mantle plume or some other cause
Answers: 3
Do you know the correct answer?
Consider the following elementary reaction: CFC13(g)-CFC12(9)+Clg) Suppose we let k1 stand for the r...
Questions in other subjects:

Social Studies, 13.11.2021 14:40







Mathematics, 13.11.2021 14:40


Health, 13.11.2021 14:40


![[Cl]_{eq}=\frac{k_1[CFCl_3]_{eq}}{k_{-1}[CFCl_2]_{eq}}](/tpl/images/0555/9861/640b9.png)