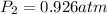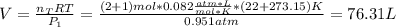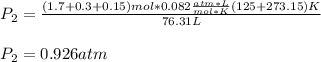A mixture in which the mole ratio of hydrogen to oxygen is (exactly) 2:1 is used to prepare water by the reaction 2 H2 (g) + O2 (g) → 2 H2O (g) The total pressure in the container is 0.951 atm at 22°C before the reaction. What is the final pressure in the container after the reaction, with a final temperature of 125°C, no volume change, and an 85.0% yield?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2


Do you know the correct answer?
A mixture in which the mole ratio of hydrogen to oxygen is (exactly) 2:1 is used to prepare water by...
Questions in other subjects:



History, 05.10.2021 08:10

Mathematics, 05.10.2021 08:10


Business, 05.10.2021 08:10

Mathematics, 05.10.2021 08:10

Physics, 05.10.2021 08:10