Chemistry, 20.03.2020 10:04, morkitus13
An ion Mn+ has a single electron. The highest energy line in its emission spectrum occurs at a frequency of 8.225 × 1016 Hz. Identify the ion. (Enter the symbol of the element in the first box, and its charge in the second.)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kkruvc
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 00:40, draveon353
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 04:00, speris1443
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3
Do you know the correct answer?
An ion Mn+ has a single electron. The highest energy line in its emission spectrum occurs at a frequ...
Questions in other subjects:



Mathematics, 08.06.2021 18:50

Mathematics, 08.06.2021 18:50


Mathematics, 08.06.2021 18:50

Biology, 08.06.2021 18:50


History, 08.06.2021 18:50

Mathematics, 08.06.2021 18:50


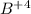
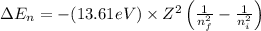
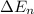 = change in energy
= change in energy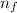 = Higher energy level =
= Higher energy level = 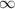
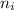 = Lower energy level = 1
= Lower energy level = 1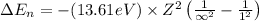
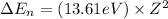 ............(1)
............(1)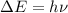
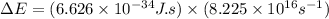
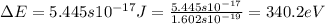 .......(2)
.......(2)