
Chemistry, 20.03.2020 10:06, masteroftheuniverse3
Solving for the pH in a mixture of acids is like dealing with a diprotic acid. You solve the problem by dealing with the acids in successive order. You start with the stronger acid (just like you start with the Ka1 because it is higher than the Ka2 for a diprotic). Afterward, you move on to the weaker acid. If a solution contains 0.029 M HCl and 0.100 M HF (Ka=6.6x10-4), what will be the pH of the solution?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 23.06.2019 09:20, annapittbull12
1) a. water molecule breaks up into hydrogen and oxygen on passing electricity. does this involve breaking intermolecular or intramolecular forces of attraction. explain b. on boiling water changes to water vapor. does this involve breaking intermolecular or intramolecular forces of attraction. explain methanol evaporates faster than water. contrast the intermolecular forces and the vapor pressures of methanol and water?
Answers: 2

Chemistry, 23.06.2019 12:00, Pointjazzyqueen602
How can nonpolar molecule contain polar covalent bonds
Answers: 1
Do you know the correct answer?
Solving for the pH in a mixture of acids is like dealing with a diprotic acid. You solve the problem...
Questions in other subjects:

Mathematics, 18.03.2021 02:10






Mathematics, 18.03.2021 02:10

English, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10

Physics, 18.03.2021 02:10


![[H^+]](/tpl/images/0555/8561/07acb.png)
![[HCl]=[H^+]=0.029 M](/tpl/images/0555/8561/09dd5.png)
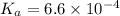
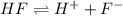
![K_a=\frac{[H^+][F^-]}{[HF]}=\frac{x\times x}{(c-x)}](/tpl/images/0555/8561/1ea44.png)
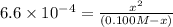
![[H^+]'=x=0.0078 M](/tpl/images/0555/8561/68e8c.png)
![[H^+]_t=[H^+]+[H^+]'=0.029 M + 0.0078 M=0.0368 M](/tpl/images/0555/8561/81922.png)
![pH=-\log[H^+]_t](/tpl/images/0555/8561/ac0ab.png)
![=-\log[0.0368 M]=1.43](/tpl/images/0555/8561/d2fa1.png)




