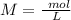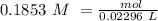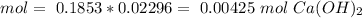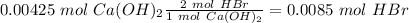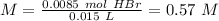Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1



Chemistry, 23.06.2019 01:00, skatelife8974
What is the chemical name of the compound ti2o3?
Answers: 2
Do you know the correct answer?
The titration of 15.00 mL of hydrobromic acid required 22.96 mL of a 0.1853 M calcium hydroxide to r...
Questions in other subjects:

English, 29.06.2019 01:30

Mathematics, 29.06.2019 01:30


Mathematics, 29.06.2019 01:30

Mathematics, 29.06.2019 01:30



Chemistry, 29.06.2019 01:30


Mathematics, 29.06.2019 01:30