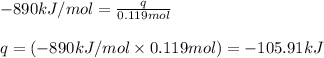Chemistry, 20.03.2020 09:52, KillerSteamcar
In the presence of excess oxygen, methane gas burns in a constant-pressure system Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure. to yield carbon dioxide and water: CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l) ΔH = -890.0 kJ Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure. Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure. Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure. Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure. Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, VictoriaRose520
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 05:00, pandasarecute53
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
Do you know the correct answer?
In the presence of excess oxygen, methane gas burns in a constant-pressure system Calculate the valu...
Questions in other subjects:




Mathematics, 02.04.2021 22:10



History, 02.04.2021 22:10



Health, 02.04.2021 22:10


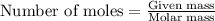
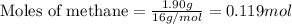
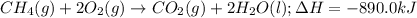
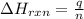
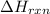 = enthalpy change of the reaction = -890.0 kJ/mol
= enthalpy change of the reaction = -890.0 kJ/mol