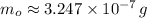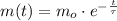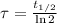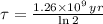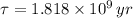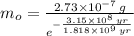Chemistry, 20.03.2020 09:44, leonardkaren41ovlx1q
Geologists use the decay of potassium-40 in volcanic rocks to determine their age. Potassium-40 has a half-life of 1.26 109 years, so it can be used to date very old rocks. If a sample of rock 3.15 108 years old contains 2.73 10-7 g of potassium-40 today, how much potassium-40 was originally present in the rock

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1



Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
Do you know the correct answer?
Geologists use the decay of potassium-40 in volcanic rocks to determine their age. Potassium-40 has...
Questions in other subjects:

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01