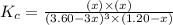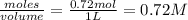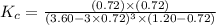Chemistry, 20.03.2020 10:09, poptropic7932
Carbon tetrachloride can be produced by the following reaction: Suppose 1.20 mol of and 3.60 mol of were placed in a 1.00-L flask at an unknown temperature. After equilibrium has been achieved, the mixture contains 0.72 mol . Calculate at the unknown temperature.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 09:30, psychocatgirl1
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone, due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
Do you know the correct answer?
Carbon tetrachloride can be produced by the following reaction: Suppose 1.20 mol of and 3.60 mol of...
Questions in other subjects:

Mathematics, 02.03.2021 19:30


Mathematics, 02.03.2021 19:30

Biology, 02.03.2021 19:30

Mathematics, 02.03.2021 19:30






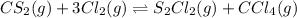
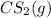 of and 3.60 mol of
of and 3.60 mol of 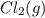 were placed in a 1.00-L flask at an unknown temperature. After equilibrium has been achieved, the mixture contains 0.72 mol of
were placed in a 1.00-L flask at an unknown temperature. After equilibrium has been achieved, the mixture contains 0.72 mol of 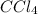 . Calculate equilibrium constant at the unknown temperature.
. Calculate equilibrium constant at the unknown temperature.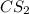 = 1.20 mole
= 1.20 mole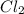 = 3.60 mole
= 3.60 mole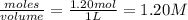
![K_c=\frac{[S_2Cl_2]\times [CCl_4]}{[Cl_2]^3[CS_2]}](/tpl/images/0555/8680/e9703.png)