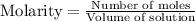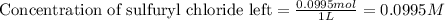Chemistry, 20.03.2020 09:55, solobiancaa
The decomposition of sulfuryl chloride into sulfur dioxide and chlorine SO2Cl2(g) → SO2(g) + Cl2(g) follows first-order kinetics. At 320◦C the rate constant is 2.2 × 10−5 sec−1 . If one started with a sample containing 0.16 moles of sulfuryl chloride per liter at 320◦C, what concentration would be left after 6.00 hours?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, matpakootas521
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2


Chemistry, 22.06.2019 10:50, adam1299
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments, solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1
Do you know the correct answer?
The decomposition of sulfuryl chloride into sulfur dioxide and chlorine SO2Cl2(g) → SO2(g) + Cl2(g)...
Questions in other subjects:


Chemistry, 25.11.2020 02:30






Arts, 25.11.2020 02:30


English, 25.11.2020 02:30


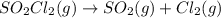
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0555/8016/f1041.png)
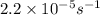
![[A_o]](/tpl/images/0555/8016/dc622.png) = initial amount of the sample = 0.16 moles
= initial amount of the sample = 0.16 moles![2.2\times 10^{-5}=\frac{2.303}{21600}\log\frac{0.16}{[A]}](/tpl/images/0555/8016/ba4a8.png)
![[A]=0.0995moles](/tpl/images/0555/8016/152d1.png)