Chemistry, 20.03.2020 09:07, khalilh1206
The following reaction was performed in a sealed vessel at 772 ∘C : H2(g)+I2(g)⇌2HI(g) Initially, only H2 and I2 were present at concentrations of [H2]=3.90M and [I2]=2.95M . The equilibrium concentration of I2 is 0.0500 M . What is the equilibrium constant, Kc, for the reaction at this temperature?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, pennygillbert
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1


Chemistry, 22.06.2019 15:00, kamkam5791
Is powdered sports drink ionic or covalent ? 10pts !
Answers: 1

Do you know the correct answer?
The following reaction was performed in a sealed vessel at 772 ∘C : H2(g)+I2(g)⇌2HI(g) Initially, on...
Questions in other subjects:


Mathematics, 25.09.2019 02:30

Biology, 25.09.2019 02:30

Health, 25.09.2019 02:30

English, 25.09.2019 02:30


Mathematics, 25.09.2019 02:30


Mathematics, 25.09.2019 02:30

Mathematics, 25.09.2019 02:30


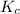 for the given equation is 160.2
for the given equation is 160.2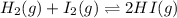
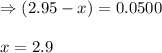
![K_c=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0555/7227/62646.png)