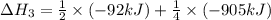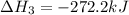Chemistry, 20.03.2020 07:19, valeriegarcia12
Nitric oxide (NO) can be formed from nitrogen, hydrogen and oxygen in two steps. In the first step, nitrogen and hydrogen react to form ammonia:
N2(g) + 3H2(g) →2NH3(g)
ΔH=−92.kJ
In the second step, ammonia and oxygen react to form nitric oxide and water:
4NH3(g) + 5O2(g) → 4NO(g) +6H2O(g)
ΔH=−905.kJ
Calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, sbhishop19
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 21.06.2019 22:00, MrSavannahCat
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1


Chemistry, 22.06.2019 21:30, kawaiiblurainbow
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
Do you know the correct answer?
Nitric oxide (NO) can be formed from nitrogen, hydrogen and oxygen in two steps. In the first step,...
Questions in other subjects:

Mathematics, 04.04.2021 02:30









Geography, 04.04.2021 02:40


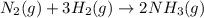 (1)
(1)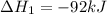
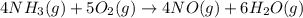 (2)
(2)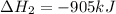
 for the following reaction i.e,
for the following reaction i.e,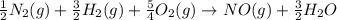
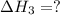
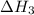 for the reaction will be:
for the reaction will be: