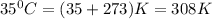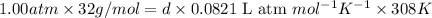Calculate the density of oxygen, O2, under each of the following conditions: STP 1.00 atm and 35.0 ∘C
Express your answers numerically in grams per liter. Enter the density at STP first and separate your answers by a comma. density at STP, density at 1 atm and 35.0 ∘C= g/L

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, sammiehammer
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 22.06.2019 00:30, VictoriaRose520
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 02:00, webbhlharryteach
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1
Do you know the correct answer?
Calculate the density of oxygen, O2, under each of the following conditions: STP 1.00 atm and 35.0 ∘...
Questions in other subjects:

Mathematics, 10.10.2019 19:30


Social Studies, 10.10.2019 19:30

English, 10.10.2019 19:30






Mathematics, 10.10.2019 19:30


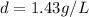 at STP,
at STP, 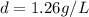 at 1 atm and
at 1 atm and 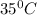

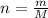

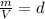 which is known as density of the gas
which is known as density of the gas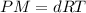

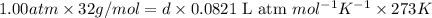
 :
: