
As a chemist for an agricultural products company, you have just developed a new herbicide,"Herbigon," that you think has the potential to kill weeds effectively. A sparingly soluble salt, Herbigon is dissolved in 1 M acetic acid for technical reasons having to do with its production. You have determined that the solubility product Ksp of Herbigon is 9.40×10−6. Although the formula of this new chemical is a trade secret, it can be revealed that the formula for Herbigon is X-acetate (XCH3COO, where "X" represents the top-secret cation of the salt). It is this cation that kills weeds. Since it is critical to have Herbigon dissolved (it won't kill weeds as a suspension), you are working on adjusting the pH so Herbigon will be soluble at the concentration needed to kill weeds. What pH must the solution have to yield a solution in which the concentration of X+ is 5.00×10−3 M ? The pKa of acetic acid is 4.76.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, kelyanthecrafte
Which of the following best defines homeostasis? forming identical cells breaking down glucose maintaining stable internal conditions increasing an organism's temperature
Answers: 3

Chemistry, 22.06.2019 00:40, draveon353
During which time interval does the object travel approximately 10 meters
Answers: 3

Do you know the correct answer?
As a chemist for an agricultural products company, you have just developed a new herbicide,"Herbigon...
Questions in other subjects:

Geography, 27.01.2021 19:00



Mathematics, 27.01.2021 19:00

English, 27.01.2021 19:00


Chemistry, 27.01.2021 19:00

Mathematics, 27.01.2021 19:00


Mathematics, 27.01.2021 19:00


![pH = -log [H_{3}O^{+}]](/tpl/images/0555/4369/a0970.png) (1)
(1)![K_{a} = \frac{[CH_{3}COO^{-}][H_{3}O^{+}]}{[CH_{3}COOH]}](/tpl/images/0555/4369/48004.png)
![[H_{3}O^{+}] = \frac{Ka*[CH_{3}COOH]}{[CH_{3}COO^{-}]}](/tpl/images/0555/4369/b87a0.png) (2)
(2) 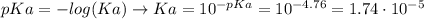
![K_{sp} = [CH_{3}COO^{-}][X^{+}]](/tpl/images/0555/4369/6ecd6.png)
![[CH_{3}COO^{-}] = \frac{K_{sp}}{[X^{+}]} = \frac{9.40 \cdot 10^{-6}}{5.00 \cdot 10^{-3}} = 1.88 \cdot 10^{-3} M](/tpl/images/0555/4369/d716a.png)
![[H_{3}O^{+}] = \frac{1.74 \cdot 10^{-5}*[1.00]}{[1.88 \cdot 10^{-3}]} = 9.26 \cdot 10^{-3} M](/tpl/images/0555/4369/f2248.png)
![pH = -log [H_{3}O^{+}] = -log [9.26 \cdot 10^{-3}] = 2.03](/tpl/images/0555/4369/7a958.png)




