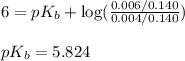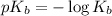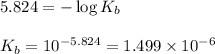Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, speris1443
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
Do you know the correct answer?
A student dissolves 0.0100 mole of an unknown weak base in 100.00 mL water and titrates the
s...
s...
Questions in other subjects:

Mathematics, 21.04.2021 02:50



Mathematics, 21.04.2021 02:50

Physics, 21.04.2021 02:50

Mathematics, 21.04.2021 02:50


Mathematics, 21.04.2021 02:50



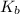 for weak base is
for weak base is 
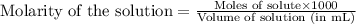
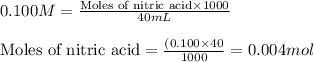
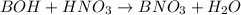
![pOH=pK_a+\log(\frac{[salt]}{[base]})](/tpl/images/0555/4585/13872.png)
![pOH=pK_b+\log(\frac{[BNO_3]}{[BOH]})](/tpl/images/0555/4585/b566f.png)
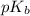 = negative logarithm of acid dissociation constant of formic acid = ?
= negative logarithm of acid dissociation constant of formic acid = ?
![[BNO_3]=\frac{0.004}{0.140}](/tpl/images/0555/4585/7cd20.png)
![[BOH]=\frac{0.006}{0.140} ](/tpl/images/0555/4585/f5b65.png)