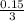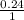Chemistry, 20.03.2020 02:58, vegeta8375
Two solutions are created by mixing one solution containing lithium nitrate with one containing sodium phosphate.
Solution A is created by mixing 0.500 L of 0.30 M LiNO3 with 0.400 L of 0.20 M Na3PO4.
Solution B is created by mixing 0.400 L of 0.20 M LiNO3 with 0.500 L of 0.30 M Na3PO4.
In which of these solutions will lithium phosphate precipitate? Lithium phosphate has a Ksp = 2.3x10−4 Select the solution(s) that will form a precipitate.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, yogibear5806
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2


Chemistry, 23.06.2019 01:00, jaidencoolman2510
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 07:30, shealynh52
Achemist at a pharmaceutical company is measuring equilibrium constants for reactions in which drug candidate molecules bind to a protein involved in cancer. the drug molecules bind the protein in a 1: 1 ratio to form a drug-protein complex. the protein concentration in aqueous solution at 25 ˚c is 1.74 x10-6 m . drug a is introduced into the protein solution at an initial concentration of 2.00 x10-6m. drug b is introduced into a separate, identical protein solution at an initial concentration of 2.00 x10-6m. at equilibrium, the drug a-protein solution has an a-protein complex concentration of 1.00 x10-6m, and the drug b solution has a b-protein complex concentration of 1.40 x10-6m. a. calculate the kc value for the a-protein binding reaction. b. calculate the kc value for the b-protein binding reaction. c. assuming that the drug that binds more strongly will be more effective, which drug is the better choice for further research?
Answers: 1
Do you know the correct answer?
Two solutions are created by mixing one solution containing lithium nitrate with one containing sodi...
Questions in other subjects:

Mathematics, 10.07.2019 19:30


Mathematics, 10.07.2019 19:30




Mathematics, 10.07.2019 19:30


History, 10.07.2019 19:30