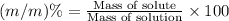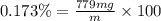Chemistry, 20.03.2020 02:35, StephenCurry34
You can practice converting between the mass of a solution and mass of solute when the mass percent concentration of a solution is known. The concentration of the KCN solution given in Part A corresponds to a mass percent of 0.173 %. What mass of a 0.173 % KCN solution contains 779 mg of KCN

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:50, namoralessimon03
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 06:30, destineedeal1
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1

Chemistry, 23.06.2019 10:00, tammydbrooks43
What is the density, d, of a substance with a volume of v = 12.5 cm3 and a mass of m = 74.4 g ?
Answers: 1

Chemistry, 23.06.2019 14:00, kitkat033157
Beaker a contains 100 ml of 1.5 mammonia. beaker b contains 100 ml of 0.50m ammonia. how does the solution in beaker a compare to the solution in beaker b?
Answers: 2
Do you know the correct answer?
You can practice converting between the mass of a solution and mass of solute when the mass percent...
Questions in other subjects:


Biology, 20.09.2019 04:30


English, 20.09.2019 04:30



Mathematics, 20.09.2019 04:30



Physics, 20.09.2019 04:30