
Chemistry, 20.03.2020 02:02, cookie42087
2.00 moles of CO2 and 1.50 moles of H2 are placed into a rigid 5.00-L container and they react according to the equation CO2(g) + H2(g) ⇄ CO(g) + H2O(g) K = 2.50 What will be the concentration of carbon monoxide when equilibrium is reached? 0.191 M 0.091 M 0.209 M (Your correct answer) 0.913 M 1.05 M

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, rosetoheart2
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 06:00, Chente379
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2


Chemistry, 22.06.2019 21:30, thompsonhomes1
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
Do you know the correct answer?
2.00 moles of CO2 and 1.50 moles of H2 are placed into a rigid 5.00-L container and they react accor...
Questions in other subjects:



Arts, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50




Mathematics, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50


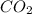 = 2.00 mole
= 2.00 mole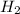 = 1.50 mole
= 1.50 mole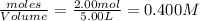
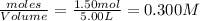
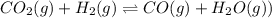
![K_c=\frac{[CO]\times [H_2O]}{[H_2]\times [CO_2]}](/tpl/images/0555/1888/1dc80.png)
![2.50=\frac{[x]\times [x]}{[0.300-x]\times [0.400-x]}](/tpl/images/0555/1888/54f3c.png)
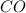 at equilibrium = x M = 0.209 M
at equilibrium = x M = 0.209 M



