
Chemistry, 20.03.2020 01:29, latinotimo7643
The combustion of propane (C3H8) in the presence of excess oxygen yields CO2and H2O:C3H8(g) + 5O2(g) 3CO2(g) + 4H2O (g)When 2.5 mol of O2are consumed in thisreaction, mol of CO2are produced.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, Gghbhgy4809
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Do you know the correct answer?
The combustion of propane (C3H8) in the presence of excess oxygen yields CO2and H2O:C3H8(g) + 5O2(g)...
Questions in other subjects:

Computers and Technology, 02.12.2020 19:40

Mathematics, 02.12.2020 19:40



Mathematics, 02.12.2020 19:40

Mathematics, 02.12.2020 19:40





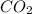 are produced.
are produced.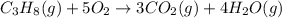
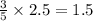 moles of carbon dioxide
moles of carbon dioxide




