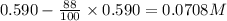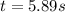Consider this reaction: →2NH3g+N2g3H2g At a certain temperature it obeys this rate law: rate =2.11·M−1s−1NH32 Suppose a vessel contains NH3 at a concentration of 0.590M. Calculate how long it takes for the concentration of NH3 to decrease by 88.0%. You may assume no other reaction is important.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:40, babygirlqueen5588
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
Do you know the correct answer?
Consider this reaction: →2NH3g+N2g3H2g At a certain temperature it obeys this rate law: rate =2.11·M...
Questions in other subjects:



English, 06.01.2021 21:10



Mathematics, 06.01.2021 21:10

Spanish, 06.01.2021 21:10




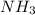 to decrease by 88.0%.
to decrease by 88.0%. 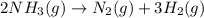
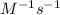 , the kinetics must be second order.
, the kinetics must be second order.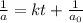
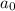 = initiaal concentration = 0.590 M
= initiaal concentration = 0.590 M