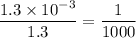Chemistry, 20.03.2020 01:06, blondielocks2002
Explain why a solution that is 1.3 M HF and 1.3 mM KF is not a good buffer. HF is a strong acid and cannot be used in a buffer system. The ratio of acid to conjugate base is outside the buffer range of 10:1. The two species are not a conjugate acid base pair. KF is not soluble in water and cannot be used in a buffer system.

Answers: 2
Other questions on the subject: Chemistry


Do you know the correct answer?
Explain why a solution that is 1.3 M HF and 1.3 mM KF is not a good buffer. HF is a strong acid and...
Questions in other subjects:


Mathematics, 17.10.2019 01:00


Biology, 17.10.2019 01:00

Health, 17.10.2019 01:00



History, 17.10.2019 01:00


Physics, 17.10.2019 01:00


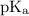 + log
+ log 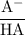
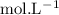 in HF and 1.3
in HF and 1.3 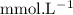 in KF, the ratio is:
in KF, the ratio is: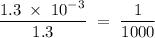
![\text{pH} = \text{pK}_{\text{a}} + \log\dfrac{\text{[A$^{-}$]}}{\text{[HA]}}](/tpl/images/0555/0502/0e377.png)
![\dfrac{1}{10} \leq \dfrac{\text{[A$^{-}]$}}{\text{[HA]}} \leq \dfrac{10}{1}](/tpl/images/0555/0502/63cff.png)